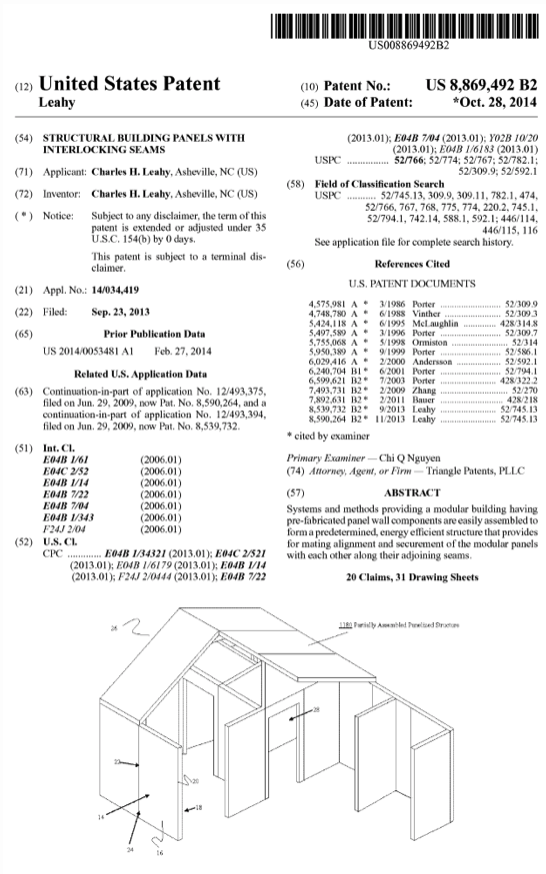
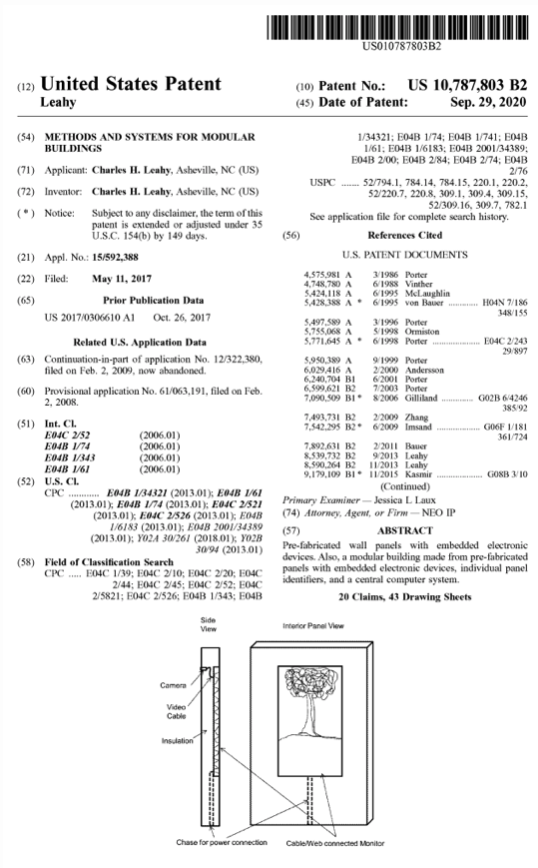
Patents & Papers
Current Patent Status

References
- Demediuk T (1952) A method for overcoming unsoundness in magnesian limes. Nature 70(4332):799
- Demediuk T, Cole WF, Hueber HV (1955) Studies on magnesium and calcium oxychlorides. Aust J Chem 8(2):215–233
- Beaudion JJ, Ramachandran VS (1978) Strength development in magnesium oxysulfate cement. Cement Concrete Res 8(1):103–112
- Yu HF (1991) Magnesium oxychloride and its application. China Building Material Press, Beijing 5. Chau CK, Li ZJ (2008) Microstructures of magnesium oxychloride. Mater Struct 41:853–862
- Chau CK, Li ZJ (2008) Microstructures of magnesium oxychloride. Mater Struct 41:853–862 6. Demediuk T, Cole WF (1957) A study on magnesium oxysulphates. Aust J Chem 10(3):287–294 7. Kahle K (1972) Mechanism of formation of magnesiumsulphate cement. Silikattecnic 23(5):148–151
- Urwong L, Sorrell CA (1980) Phase relations in magnesium oxysulfate cements. J Am Ceram Soc 63(9–10):523–526 9. YuHF (1992)Study ofnew typewater-resisting magnesium oxychloride cement. J Chin Ceram Soc 20(4):1203–1205 10. Li HQ, Li GZ, Yu YZ (2008) The influence of compound additive on magnesium oxychloride cement/urban refuse floor tile. Constr Build Mater 22(4):521–525
- Deng DH (2003) The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem Concr Res 33(9):1311–1317
- Griffin JLW, Coveney PV, Whiting A (1999) Design and synthesis of macrocyclic ligands for interaction with crystalline ettringite and demonstration of available mechanism for the setting of cement. J Chem Soc Perk Trans 110(2):1973–1981
- Ramachandran VS, Lowery MS, Wise T, Polomark GM (1993) The role of phosphonates inthehydration of Portland cement. Mater Struct 26(161):425–432
- Billingham J, Francis DTI, King AC et al (2005) A multiphase model for the early stages of the hydration of retarded oilwell cement. J Eng Math 53(2):99–112
- Black SN, Bromley LA, Cottier D et al (1991) Interactions at the organic inorganic interface-binding motifs for phosphonates at the surface of barite crystals. J Chem Soc Faraday Trans 87(20):3409–3414
- Al Rohl, Gay DH, Davey RJ, Catlow CRA (1996) Interactions at the organic/inorganic interface: molecular modeling of the interaction between diphosphonates and the surfaces of barite crystals. J Am Chem Soc 118(3):642–648
- Wallner CE (2010) Cementitious veneer useful for forming laminated tiles/panels in building applications comprises magnesium oxysulfate compound comprising e.g. magnesium sulfate; and cementitious composition comprising e.g. hydrate calcium sulfate. USA patent US2010222457-A1; US8182605-B2
- Kishimoto T, Yamamoto S (2009) Modified magnesium oxysulfate fibrous particles, useful e.g. in polyolefin composition, comprises magnesium oxysulfate fibrous particles treated on their surfaces with nucleating agent comprising e.g. phosphoric acid compounds. USA patent US200929 2047-A1
- Birchal VSS, Rocha SDF, Ciminelli VST (2000) The effect of magnesite calcination conditions on magnesia hydration. Miner Eng 13(14–15):1629–1633
- Li ZJ, Chau CK (2008) Reactivity and function of magnesium oxide in Sorel cement. J Mater Civil Eng 20(3):239– 244
- Dong JM, Yu HF, Zhang LM (2010) Study on experimental conditions of hydration methods of determining active magnesium oxide content. J Salt Lake Res 18(1):38–41
- Bishop M, Bott SG, Barron AR (2003) A new mechanism for cement hydration: solid-state chemistry of calcium nitrilotris(methylene)triphosphonate. Chem Mater 15(16): 3074–3088 23. Saloma ˜o R, Pandolfelli VC (2011) Citric acid as antihydration additive for magnesia containing refractory castables. Ceram Int 37(6):1839–1842